Freedom of Information Act
Defending the Republic Obtains Troubling Moderna
Documents in its FOIA Lawsuit Against the FDA
Defending the Republic has just received thousands of pages of Moderna COVID-19 vaccine documents, including materials relating to serious adverse events and post-vaccine deaths, Moderna responses to FDA concerns, spreadsheets detailing potential vaccine injuries, and summaries of meetings between the FDA and Moderna.
These documents were provided to Defending the Republic as part of our Freedom of Information Act (FOIA) lawsuit against the FDA. The FDA fought the expedited release of these documents until Defending the Republic filed suit.
This constitutes the largest public release of Moderna’s COVID-19 vaccine records to-date. In order to further the public’s understanding of this vaccine and the FDA’s questionable “approval” practices, Defending the Republic has made them available for download below.
Please support our efforts to expose government corruption, promote government accountability and transparency, fight COVID-19 mandates on behalf of military servicemembers, combat censorship, and to restore integrity in our elections.
Here are just some of the key findings and takeaways from these records:
- On January 24, 2022 (approximately one week before the FDA announced its approval of Spikevax), Moderna informed the FDA that it had “no plan to produce BLA [biologics license application] SPIKEVAX labelled material.” This is significant for a number of reasons. First, BLA vaccines are subject to stricter testing and safety standards than Emergency Use Authorization (EUA) vaccines. Second, United States armed service members were mandated by Secretary of Defense Lloyd Austin to receive COVID-19 vaccines; he could only issue this order if the vaccines were “FDA approved.” However, the FDA (and the Department of Defense) knew that Moderna would not be providing the “approved” vaccines to the military. Third, it misled the public that they were getting “approved” vaccines while actually receiving EUA vaccines approved under lesser standards.
- Moderna records revealed the trial participants that acquired shingles (Herpes Zoster) soon after receiving the vaccine. For example, a 49 year-old female test subject had shingles approximately 10 days after receiving the second shot of the Moderna vaccine. Another participant, a 67 year-old female, was diagnosed with shingles only 3 days after receiving the first Moderna shot.
- In one table submitted to the FDA, Moderna concluded that the vast majority of “Adverse Events of Herpes Zoster” were not related to its vaccine. Exactly how those suspicious and self-serving determinations were made is unknown.
- The FDA noted “an imbalance of herpes zoster cases in the mRNA-1273 arm versus the placebo arm.”
- For trial participants who received the Moderna COVID-19 vaccine, “The majority of [herpes zoster] events occurred after the 1st dose (43.9%) with 19.3% of all events occurring <7 days after the first dose.”
- One Moderna spreadsheets submitted to the FDA indicated a disturbing number of “less serious” adverse events within 10 days of vaccination, including fever, vomiting, chills, and gland swelling.
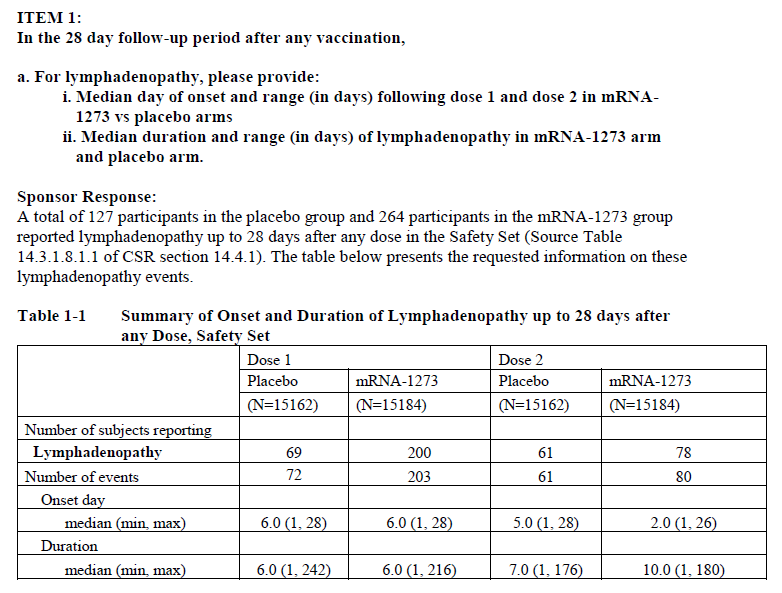
- Spreadsheets detailing trial participants who received the Moderna vaccine and developed swelling of the lymph nodes. There were excess reports of lymphadenopathy (swollen lymph nodes) as compared to the placebo.
- Inadequate investigations of adverse event symptoms by Moderna during the BLA submission. Moderna would advise the FDA on December 16, 2021 (just six weeks before Spikevax was “approved”) that thousands of ongoing adverse event symptoms had “no investigator assessment” contrary to FDA guidelines: “1892 of 6721 ongoing symptoms have no investigator assessment.”

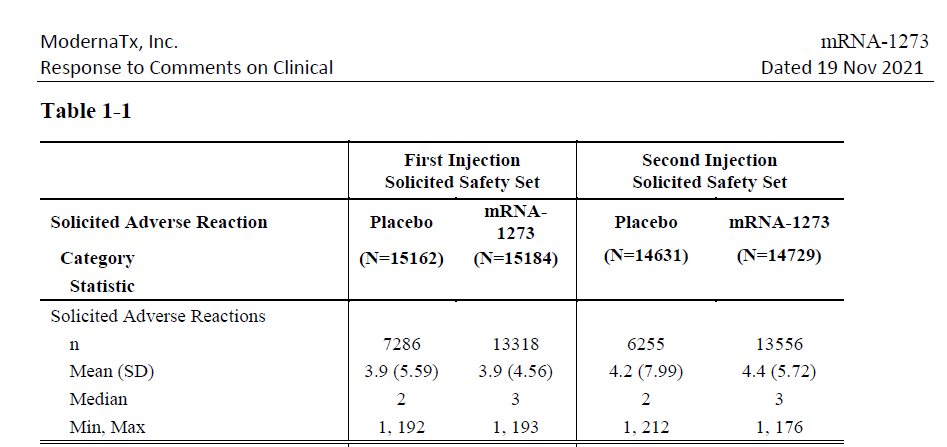
- The number of total adverse reactions after receiving the Moderna COVID-19 vaccine was almost double that of the placebo.
- A number of heart attacks (myocardial infarction) occurring days after vaccination.
This is just the summary – the source documents themselves are much more troubling. As we fight for transparency, please support our efforts to expose government corruption, promote government accountability through FOIA lawsuits, fight COVID-19 mandates and misinformation, combat censorship, and to restore integrity in our elections.
Download the Moderna Files by File or Category:
Moderna Responses to FDA Clinical Comments (includes information on adverse events and deficiencies in Moderna’s submissions to the FDA)
Dec 10 2021 IR#30 and Adverse Events and Bells Palsy
Dec 10 2021 IR#32
Dec 16 2021 IR#35
Dec 16 2021 IR#36 and Adverse Events
Dec 16 2021 IR#37 and Adverse Events
Dec 17 2021 IR#39 and Adverse Events
Jan 13 2022 IR#45 and Adverse Events
Jan 26 2022 IR#48 and Adverse Events
Nov 10 2021 Safety – Herpes Zoster
Nov 19 2021 Datasets
Nov 30 2021 IR #24 and efficacy
Oct 22 2021 Adverse Events
Oct 22 2021 Formulation
Oct 28 2021 IR#5 and Safety
Oct 29 2021 IR #12
Sept 14 2021 BLA IR#1
Sept 14 2021 FDA BLA IR#1
Sept 14 2021 FDA Clinical Comments
Sept 17 2021 IR#2
Sept 17 2021 Moderna Response to FDA Clinical Comments
Sept 22 2021 Moderna Response to FDA Info Request
Sept 23 2021 Moderna Response to FDA Clinical Comments re Formulation
Sept 23 2021 Moderna Response to FDA Clinical Comments
Sept 24 2021 Moderna Response to FDA re Adverse Events
Sept 24 2021 Moderna Response to FDA reAdverse Events and Groupings
Sept 28 2021 Moderna Response to FDA Clinical Comments
Meeting Minutes (includes discussion that Moderna did not plan to produce Spikevax)
Jan 24 2022 Meeting Minutes from FDA and Moderna Meeting
Clinical Trial Information (includes information on adverse events and efficacy)
CBER Requested Tables – Safety and Adverse Events
CBER Requested Tables Batch 2
CBER Requested Tables
Moderna – Mapping
Tables – Summary of Deep Vein Thrombosis up to 28 days post injection
Tables – Summary of Dyspnoea up to 7 days post injection
Tables – Summary of Syncope up to 7 days post injection
Moderna Excels and Data (includes data on adverse events such as facial paralysis, Herpes Zoster, vertigo, and Lymphadenopathy)
0015152 to -0015310_125752_S20_M1_l-ae-solicited-term-by-subj-exarm-2021-05-04
0015311_125752_S20_M1_taug-vax
0015456_ 125752_S33_M1_facial-paralysis
0015457_125752_S33_M1_herpes-zoster-rtq-cber
0015458 to -0015459_125752_S33_M1_hypersensitivity-7days
0015460 to -0015464_125752_S39_M1_vertigo
0015465_125752_S40_M1_deep-vein-thrombosis
0015466 to -0015469_125752_S40_M1_dyspnoea-syncope
0015470 to -0015474_125752_S40_M1_ir-37-item-3-ceday8
0015475 to -0015479_125752_S40_M1_ir-37-item4-after7
0015480_125752_S40_M1_ir-37-item4-missing-aesofl
0015481 to -0015482_ 125752_S40_M5_table-14-3-1-37-3-1
0015483 to -0015484_125752_S40_M5__table-14-3-1-37-3-2
0015485 to -0015486_125752_S40_M5__table-14-3-1-37-4
0015487 to -0015492_125752_S44_M1_rsp-fda-on-info-req43-07jan2022
Spikevax Inserts (Draft “Highlights of Prescribing Information” including FDA edits)
Moderna Spikevax Insert Draft – Jan 27 2022
Moderna Spikevax Insert Draft – Jan 28 2022
Moderna Spikevax Insert Draft with FDA redlines – Jan 27 2022
Moderna Spikevax Insert Draft with FDA redlines – Jan 28 2022
Letters (Moderna letters to the FDA discussing the production of materials)
Dec 2 2021 Moderna re IR#24
Dec 10 2021 Moderna re Updated Datasets
Dec 13 2021 Moderna re IR#30
Dec 17 2021 Moderna re IR#32
Dec 20 2021 Moderna re IR#36 IR#37 IR#39
Dec 20 2021 Moderna re IR#36
Jan 10 2022 Moderna re BLA and IR#43
Jan 14 2022 Moderna re BLA and IR#45
Jan 24 2022 Moderna re IR#47 and Adverse Events
Jan 25 2022 Moderna re IR#47
Jan 26 2022 Moderna re Launch Lots
Jan 27 2022 Moderna re IR#48
Jan 28 2022 Moderna re IR#49
May 28 2021 Moderna re BLA
Nov 5 2021 Moderna re Meeting Minutes and Follow Up
Nov 9 2021 Moderna re Effectiveness Study Protocol
Nov 16 2021 Moderna re IR#19
Nov 30 2021 Moderna re SDTM Datasets for IR#23
Oct 4 2021 Moderna re Info Requests
Oct 8 2021 Moderna re Tables and IR#6
Oct 13 2021 Moderna re IR#7
Oct 19 2021 Moderna re BLA
Oct 25 2021 Moderna re IR#5
Sept 15 2021 Moderna re CBER Requested Tables Supporting BLA
Sept 21 2021 Moderna re Info Request
Sept 27 2021 Moderna re Info Request
Sept 29 2021 Moderna Letter to FDA re Tables and IR#4
Research (Kaiser Permanente Effectiveness Study)
Kaiser Permanente – Real-World Study of the Effectiveness of Moderna COVID-19 Vaccine – Sept 2021
Forms
HHS-FDA Certification of Compliance
HHS-FDA Prescription Drug User Fee Coversheet FY 2021
Full Download of All Moderna Documents